To view this post on my blog site, sign up for future posts, and be able to search for previous posts, please click here.
The presentation that received the most attention from readers of this blog and the press at this year’s ASCO meeting was the one about Lu-177-PSMA-617 for patients with advanced, metastatic castrate resistant prostate cancer (mCRPC). I have previously posted about PSMA and this approach to treatment as you may want to review here. Briefly, Prostate Specific Membrane Antigen, is a protein expressed on the surface of prostate cancer cells. There are molecules (ligands) that bind to this protein and can be tagged with radioactive isotopes. Thus, the tagged ligand, once injected, carries the isotope to the tumor cells. If the isotope is a positron emitter, a CT-PET scanner (Positron Emission Tomography) will light up the tumor’s location. Examples include Ga-68 and F-18. If the isotope releases stronger radiation, (for example Lu-177 releases strong beta particles that can kill cancer cells, just as the approved agent, Radium 223 -aka Xofigo™ -is a bone seeking agent that seeks out bone metastases and kills cancer cells by releasing strong alpha particles) then prostate cancer cells expressing PSMA will be killed.
The data presented at ASCO 2021 on Lu-177-PSMA-617 was from a large phase III trial comparing Lu-177-PSMA-617 with “standard of care” in patients who had progressed on most other therapies. The results are shown in the following figure:
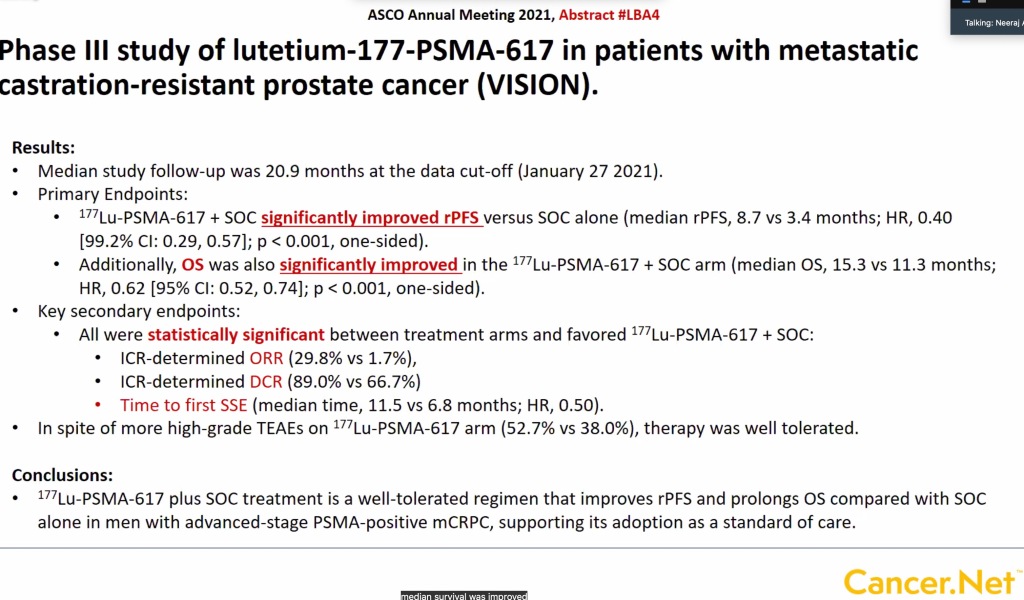
These data will be evaluated by the FDA and it seems likely this new therapy will be approved. The answer to the question of “what’s next?” for a new drug is usually to study its use in earlier stages of disease. What if patients who have metastases but have not yet been treated with hormonal manipulation were to receive the drug at the same time they start hormonal treatment? What if used before prostatectomy? There are 9 such ongoing trials you can read about here. The hope is, that by using the drug earlier, even more benefit will result, and this is often the case in cancer medicine – for example using early “adjuvant” chemotherapy in high risk breast cancer, or using apalutamide (Erleda™) at the outset when initiating prostate cancer ADT in high risk patients.
As we progress in our understanding of when and in whom to use more aggressive therapies, it will also be helpful to identify the patients at greatest risk for failing one treatment or another. In an article appearing this month in Annals of Oncology, investigators evaluated tumor DNA levels after a single cycle of abiraterone (Zytiga™) and found that patients who didn’t have circulating tumor DNA at the start or converted from positive to negative had significantly better overall survival than patients who did not convert to negative. This means that as soon as 30 days after starting abiraterone, you could already pick out patients in whom you might want to change therapy or add other agents to treatment. They also showed that patients with alterations in specific genes like TP53, RB1 or PTEN either at pretreatment or after one cycle had significantly shorter overall survival. This kind of individualizing risk analysis will further enhance the ability to introduce new drugs like Lu-177-PSMA-617 earlier in patients who need it and avoid toxicities in those who don’t.
For those who helped support my mustache during Movember, these findings are tangible evidence of real progress we can all be proud of. You can share in the great feelings and read about your accomplishment here: https://au.movember.com/story/new-treatment-for-men-with-advanced-prostate-cancer-more-effective-than-chemotherapy?tag=prostate-cancer. Our donations DO make a difference and thanks for your help!




Mike, This is very interesting and you helped us understand some of confusing stuff in the popular press. One question I can’t seem to find answers for is using beta radiation in some critical tissues/areas. Often we have our mets in periaortic nodes. Is that amount of radiation dangerous close to the aorta or other major organs?
I am hoping you might help us understand the newly approved imaging agent , Pylarify. Especially comparing it to the standard FDG PET/CT that most of us are getting. Is this going to help define tumor burden better? What is the likelihood we will see it in places like Colorado?
Thanks as always for helping us understand.
Bob
FDG pet generally doesn’t work very well in prostate cancer. Pylarify is the PSMA directed PET agent, now approved. I have posted about it – search on “psma” on the blog. My understanding is that they are rolling it out in the US asap. I would expect colorado nuc med departments to have it within 2-3 months. Yes, it is much better than standard imaging (CT, Bone scans) and also 2-3x more sensitive than the previously approved “axumin” scan (fluciclovine).
The beta radiation is very short (can be stopped by a piece of paper). Toxicities are very localized and only in organs that take up the drug as a normal process. For PSMA-Lu177, this is mostly the salivary glands. One would expect no damage to lymph node areas. If someone has extensive bone mets, it could suppress white count,
From: prost8blog
Reply-To: “comment+r1s9kl4sim04t5mes_m6q48m64p@comment.wordpress.com”
Date: Wednesday, June 16, 2021 at 3:23 PM
To: “Glode, Mike”
Subject: [prost8blog] Comment: “Lu-177-PSMA-617 and “what’s next?””
Is this available now on an emergency exception basis
I don’t know. I think you would have to ask the FDA, but also the company making the product needs to allow. Often they don’t release it except in studies unless/until there is an FDA approval. You could contact company most easily to find out. Then you would probably still need to go to an institution that already has approval to administer as part of a trial.
Pingback: Join me fighting prostate cancer in the “Hairiest month of the Year!” | prost8blog