To view this on my blog site and subscribe to future posts, click here.
The big news at the ASCO annual meeting this year included two “practice changing” presentations for prostate cancer that have been widely covered in the medical press. The basic finding is that adding abiraterone to ADT in the “up front” setting (i.e. when initially starting ADT for high risk disease, a rising psa or in someone who has newly diagnosed metastatic disease) results in markedly improved overall survival and time to progression of disease. I encourage you to read the link above which reviews the presentations and commentaries in detail. Reflecting our amazing digital age, the New England Journal published the detailed, peer-reviewed articles on the two studies simultaneously with the ASCO presentations.
In the Latitude trial, 1199 men who were newly diagnosed with metastatic disease and who “… were considered to have at least two high-risk prognostic features (e.g., a Gleason score of ≥8, the presence of ≥3 bone lesions, or the presence of measurable visceral metastasis)” were randomized to receive a GnRH analog plus abi/prednisone vs GnRH analog plus dual placebos. The science behind this is that with surgical (orchiectomy) or medical (GnRH analog) castration, testosterone synthesized by the testicles is mostly eliminated. But there is still stimulation of the prostate cancer androgen receptors (AR) by other testosterone, coming from the adrenal glands, the tumor cells making testosterone themselves, other steroids, or even changes in the androgen receptor itself. Abiraterone or its metabolites can block most of these pathways. Thus the question is whether nearly complete shutdown of AR stimulation from the onset of treatment can achieve more than waiting to use Abi after resistance to AR directed treatment with a GnRH analog develops. The results were remarkable:
A second trial, from the STAMPEDE group used a similar approach in a different, more heterogeneous group of 1917 patients with high risk localized disease, metastatic disease, or PSA relapsing disease after local therapy. The randomization to standard ADT alone vs ADT plus Abi/prednisone was similar as were the remarkably positive outcomes.
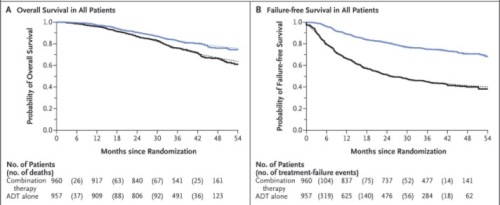
The P-values for some of the endpoints in this trial were eye-popping. “Time to failure improved by 71% in the abiraterone group (HR 0.29, 95% CI [0.25, 0.34]; p = 0.377×10-61)” The discussants at ASCO pointed out that future trials may need to consider comparison of abiraterone early addition to the early addition of chemotherapy as was done in the CHAARTED trial. Generally, however, the toxicities of docetaxel are certainly greater than abiraterone and such a trial would be complicated. It is even possible that one of the ways docetaxel works is “simply” blockade of AR translocation and that it is just a more toxic way of hitting the same pathway. In addition, the cost considerations of abiraterone, particularly when it must be taken for years, are a real concern, and in keeping with the very keen worries we have regarding new cancer drugs in general.
“Targeted therapies” along with the immune checkpoint blockade drugs are changing the landscape for cancer patients. To me, the somewhat surprising thing about these two studies is how great the advantage seems to be when you can truly effectively hit a target early and hard, in this case the AR signaling pathway. It is all the more remarkable that this is a pathway we have now been targeting for over 70 years and there is still more to come. If we can find the right combination of AR targeting and immune modulation, I see no reason why metastatic prostate cancer can’t be added to the “curable neoplasm” list in the near future. Then the question of whether, and how we need to screen will become even more complex. Good news !!
Disclaimer: I am a consultant for Janssen, the pharmaceutical company that sponsored the Latitude trial discussed above.






Thanks again for your timely and concise summary of this new data. Please now do the same for the phase 2 combo of PROVENGE and INDOXIMOD! Prost8Blog continue to be one of my go-to sources for prostate cancer treatment updates.
CAN we talk about the COST. $9200 for a months dosage! The profit making pharmaceutical companies! John currently taking it!
Hi Michael,
Healthline would like to congratulate you on making our list of the Best Prostate Cancer Blogs of 2017!
Our editors carefully selected the most up-to-date, informative, and inspiring blogs that aim to uplift their readers through education and personal stories. We’re glad to have you on the list!
We’ve created a badge that you can embed on your site to let your readers know about your win. The embed code is at the link below.
Winners list: http://www.healthline.com/health/prostate-cancer/best-blogs-of-the-year
Badge to embed: http://www.healthline.com/health/prostate-cancer/best-blogs-badge-2015
If you have any questions or need help embedding the badge, feel free to be in touch. Congratulations and keep up the great blogging!
Warmly,
Maegan
—
Maegan Jones | Content Coordinator
Healthline
Your most trusted ally in pursuit of health and well-being
Thanks. However, your list seems to refer to me as a patient who had advanced metastatic disease etc. That is not me, but a urologist I blogged about. I am a treating physician and you can read about me on the “about” link.
Of course you are a consultant for Janssen. Reading it only made me sadder. Disgraceful how my husband who desperately needs this as his last chance after going thru every possible treatment for 11 years, can’t get it because of the cost. I think Janssen is heartless and cruel.
Pingback: Rising PSA – When to start therapy | prost8blog
Pingback: Are we any closer to cure? (yes and no) | prost8blog
Pingback: An Amateur Explanation of Immunotherapy | prost8blog
I’ve been on Abiraterone for about a year, and it’s working well. My PSA has been <0.1 since taking the drug. Soon after learning how well it worked for me, I cycled 4,200 miles across the US in the summer of 2018 and raised over $1 a mile for prostate cancer research. (See touringonthatbike.com for more info)
I'll get to my question. What do we know about how long Abiraterone stays affective?
I seem to be similar to the men in the Lattitude and Stampede Trail that had no spread of the disease at the end of those studies.
If I understand right, Latitude lasted 44 months, and Stampede lasted 54 months. Has anyone kept following the men in those studies to see how long Abiraterone was or is effective for them?
I know other drugs are available for me when abiraterone stops working. I would just like to have an estimate of how long it might work.
Thanks,
Steven
Steven, This is the last information I can find re the Latitude trial. It is from an abstract presented at the ASCO meeting in June.
Background:
The LATITUDE study in pts with NDx-HR mCNPC found significant improvements in OS, rPFS, and all secondary end points, including pt-reported outcomes when combining AA + P with androgen deprivation therapy (ADT) vs placebos (PBOs) + ADT, which led to unblinding of the study at first interim analysis.
Methods:
1199 NDx-HR mCNPC pts were randomized 1:1 to AA (1 g QD) + P (5 mg QD) + ADT or PBOs + ADT. Co-primary end points were OS and rPFS. A stratified proportional hazards model assessed longer term follow-up on OS, secondary end points, and adverse events (AEs), including those associated with mineralocorticoid excess, from the preplanned second interim analysis, when approximately 65% (≈554) of expected deaths were observed.
Results:
With a median follow-up of 41 mo (range, 0.1-54.0), 205 (34%) pts in the AA + P arm and 70 (12%) in the PBOs arm (of whom 57 [81%] had crossed over to AA + P) remained on treatment. Updated OS continued to favor the AA + P arm (HR [95% CI] 0.638 [0.538-0.758]; p < 0.0001) as did secondary end points (Table). Serious AEs occurred in 27% of pts on AA + P and 20% of pts on PBOs. Grade 3/4 AEs included (AA + P vs PBOs; %): hypertension (21 vs 10), hepatotoxicity (8 vs 3), hypokalemia (12 vs 2), fluid retention (1 vs 1), and cardiac disorders (4 vs 1). 328 pts (54%) from the PBOs arm received a life-prolonging therapy.
Conclusions:
This long-term analysis continues to demonstrate the OS benefit of adding AA + P to ADT in NDx-HR mCNPC pts, with a 36% reduction in the risk of death, although most pts remaining on PBOs treatment had crossed over to AA + P. The secondary end points also continued to favor the AA + P arm, underscoring AA + P as a standard of care for pts with NDx-HR mCNPC. Clinical trial information: NCT01715285
Thanks!
Pingback: Why can’t we cure this??? | prost8blog